Organic solvents are widely used in chemistry and chemical industry due to their ability to dissolve a variety of substances, especially organic compounds.
Content
Here are some sections, we will discuss in this tutorial.
- Definition of Organic Solvents
- Applications of Organic Solvents
- Key Characteristics of Organic Solvents
- Common Types of Organic Solvents
- Selecting and Selection Criteria of Correct Organic Solvent

Definition of Organic Solvents
Organic solvents are liquids primarily composed of carbon-containing molecules used to dissolve, suspend, or extract other substances, particularly organic compounds.
Applications of Organic Solvents
They are essential in many industrial, laboratory, and chemical processes because they can dissolve a wide range of materials that are not typically soluble in water.
Key Characteristics of Organic Solvents
Selecting the correct organic solvent depends largely on the characteristics of the solvent and how they align with the desired chemical process.
Carbon-based Structure
Organic solvents are mostly made up of carbon and hydrogen atoms, often with functional groups like -OH, -C=O, or -Cl.
Due to these different functional groups, organic solvenets have different properties. As an example, methanol is soluble in acitic acid (which shows acidic properties in water) , but methanol is not soluble in cyclohexane.
Volatility and Evaporation

Many organic solvents are volatile, meaning they easily evaporate at room temperature and volatality increases with the temperature.
Extracting chemicals by evaporation
This concept is widely used to dissolve some valubale chemicals in the solvent and followed by evaporating the solvent. At the end of the evaporation, required chemical is extracted from the solvent.
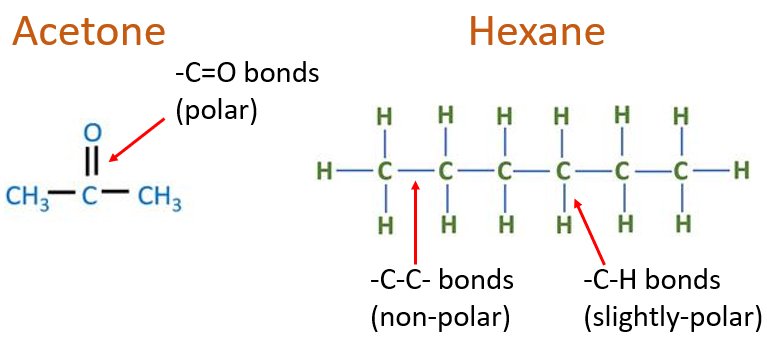
Polarity Range of Organic Solvents
Organic solvents vary in polarity, from non-polar (e.g., hexane) to polar aprotic (e.g., acetone) and polar protic (e.g., ethanol), allowing them to be matched with different solute properties.

Examples for Non-polar organic solvents
Hexane, Heptane, Benzene, Toluene, Diethyl ether are few examples for non-polar organic solvents.
Examples for Polar organic solvents
As examples, Acetone, Dichloromethane, Dimethyl sulfoxide, Acetonitrile and more are used.
Common Uses and Applications
They are used in chemical synthesis, cleaning, extractions, chromatography, and as carriers for paints and coatings.

Seaweed Extration as an application
Seaweed extraction using chemical extraction involves treating dried seaweed with solvents (e.g., ethanol, methanol) to isolate bioactive compounds like polysaccharides, proteins, and antioxidants. The seaweed is soaked, agitated, and filtered, then the solvent is evaporated to retrieve concentrated extracts, commonly used in food, pharmaceuticals, and cosmetics.
Cleaning and Degreasing
Solvents like acetone, isopropanol, and methanol are effective for removing oils, grease, and contaminants in industries and labs.
Common Types of Organic Solvents
Non-Polar Solvents
- Hexane: Commonly used for extractions and chromatography due to its low polarity.
- Toluene: Used in reactions and as a paint thinner. It also has applications in spectroscopy.
- Benzene: Historically used as a solvent, though its use is now restricted due to toxicity, specially because of being a carcinogenic suspicious.
Polar Aprotic Solvents
- Acetone: Highly versatile, often used in cleaning, as a reagent, and in extractions.
- Dichloromethane (DCM): Widely used for extractions and as a solvent in organic synthesis, though it’s toxic and volatile.
- Dimethyl sulfoxide (DMSO): Known for its ability to dissolve both polar and non-polar compounds; used in biological studies and reactions.
- Acetonitrile: Used in HPLC (high-performance liquid chromatography) and organic synthesis.
Polar Protic Solvents
- Methanol: Commonly used in organic reactions, extractions, and as an antifreeze agent.
- Ethanol: Frequently used in biological and organic chemistry; serves as both a solvent and a cleaning agent.
- Isopropanol (Isopropyl alcohol): Used in reactions, extractions, and as a disinfectant.
Halogenated Solvents
- Chloroform: Previously used widely for extractions but now less common due to toxicity.
- Carbon tetrachloride: An effective solvent for certain organic compounds but is highly toxic and carcinogenic.
Ethers
- Diethyl Ether: A common solvent for extractions and reactions, though highly flammable.
- Tetrahydrofuran (THF): Widely used in polymer chemistry and organic synthesis; it is miscible with water.
Aromatic Solvents
- Xylene: Used as a solvent in paints, inks, and lab applications. Xylene has identified as a carcinogenic chemical and needs a special care to avoid and minimize the exposure.
Each of these solvents has different applications depending on the chemical properties required by the reaction or process, such as polarity, boiling point, or reactivity.
Selecting and Selection Criteria of Correct Organic Solvent
Selecting the correct organic solvent depends largely on the characteristics of the solvent and how they align with the desired chemical process. Here’s a breakdown of the most critical characteristics of organic solvents and how they influence solvent selection:
Polarity
- Effect on Solubility: The solvent’s polarity determines its ability to dissolve polar or non-polar substances. Non-polar solvents (like hexane) are chosen for dissolving non-polar compounds, while polar solvents (like ethanol) are better for polar compounds.
- Influence on Reactions: Polar aprotic solvents (e.g., acetone) are preferred for nucleophilic substitution reactions, whereas polar protic solvents (e.g., methanol) can participate in hydrogen bonding and stabilize ions, influencing reaction pathways.
Boiling Point and Volatility
- Effect on Evaporation Rate: Solvents with lower boiling points (e.g., diethyl ether) evaporate quickly, which is helpful in processes where the solvent must be removed easily. Higher boiling points (e.g., DMSO) are preferable for reactions that need prolonged heating.
- Impact on Reaction Control: Volatile solvents are used in reactions requiring rapid solvent removal, such as recrystallization or solvent casting.
Reactivity
- Chemical Stability: Solvents must be inert under reaction conditions. For example, diethyl ether is non-reactive in most organic reactions, while acetone may undergo reactions with strong bases or nucleophiles.
- Role as Catalyst or Reactant: Some solvents may also serve as reactants (e.g., methanol in esterification) or interact with the catalyst, impacting reaction yields and selectivity.
Toxicity and Safety
- Health and Environmental Concerns: Many solvents are toxic, flammable, or environmentally hazardous. For example, DCM (dichloromethane) is effective but toxic, so less hazardous alternatives like ethyl acetate may be chosen when possible.
- Compliance and Disposal: Regulatory guidelines often dictate solvent choices, particularly in pharmaceuticals or food industries, where safe, low-toxicity solvents are preferred.
Cost and Availability
- Budget Considerations: Solvents like ethanol and acetone are widely available and inexpensive, making them common choices in research and industrial applications. Specialty solvents are reserved for specific needs due to cost.
Latest Posts

